Delving into the fascinating realm of energy ws #1 reaction rates, we embark on a journey to unravel the intricacies of chemical reactions and their dependence on energy. This exploration promises to shed light on the fundamental principles that govern the speed and efficiency of chemical transformations.
As we delve deeper, we’ll uncover the factors that influence reaction rates, such as temperature, concentration, and the presence of catalysts. We’ll also investigate the concept of activation energy and its pivotal role in determining the likelihood of reactions occurring.
Reaction Rates
Reaction rates describe how quickly a chemical reaction proceeds. They quantify the change in concentration of reactants or products over time.
To grasp the intricacies of energy ws #1 reaction rates, it’s crucial to understand the mechanisms of sensory perception. Just as our ears allow us to perceive sound, when we hear something, we employ the cuando oyes algo usas el , a vital organ responsible for hearing.
This understanding provides a foundation for delving deeper into the factors influencing energy ws #1 reaction rates, enabling us to optimize and control these processes effectively.
Factors influencing reaction rates include:
- Concentration:Higher concentrations lead to more frequent collisions and faster reactions.
- Temperature:Higher temperatures increase the kinetic energy of reactants, leading to more collisions and faster reactions.
- Surface area:Increased surface area provides more contact points for reactants, leading to faster reactions.
- Catalysts:Catalysts provide alternative pathways for reactions, lowering the activation energy and speeding up reactions.
- Inhibitors:Inhibitors block or slow down reactions by interfering with the activation process.
Methods to Measure Reaction Rates
Reaction rates can be measured using various methods, including:
- Concentration changes:Measuring the change in concentration of reactants or products over time using techniques like spectrophotometry or titrations.
- Gas evolution:Measuring the volume or rate of gas produced in reactions involving gases.
- Temperature changes:Monitoring temperature changes in exothermic or endothermic reactions.
- Radioactive decay:Using radioactive isotopes to track the rate of radioactive decay.
- Spectroscopy:Analyzing changes in the absorption or emission spectra of reactants or products to monitor reaction progress.
Energy and Reaction Rates
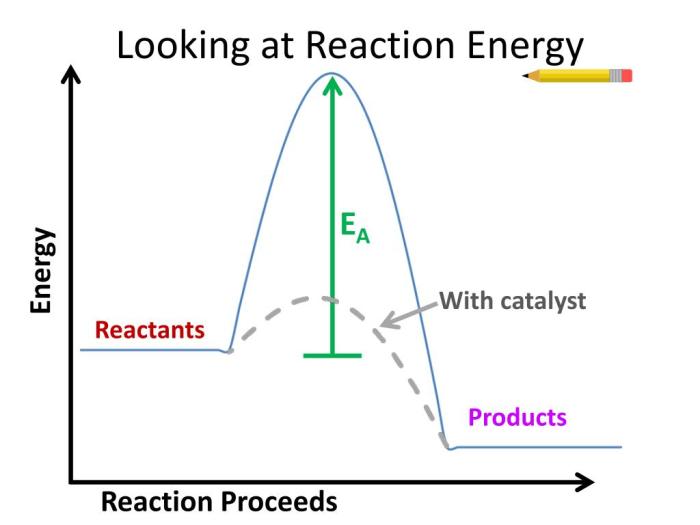
Energy plays a crucial role in determining the rate at which chemical reactions occur. The relationship between energy and reaction rates can be explained in terms of activation energy, which is the minimum amount of energy that reactants must possess to overcome the energy barrier and form products.
Activation Energy
Activation energy is like a threshold that reactants must cross to initiate a reaction. The higher the activation energy, the slower the reaction rate. Conversely, a lower activation energy leads to a faster reaction rate. This is because reactants with higher energy are more likely to possess the necessary energy to overcome the activation energy barrier and react.
Examples of Energy’s Impact on Reaction Rates
There are numerous examples of how energy can affect reaction rates:
- Temperature:Increasing the temperature of a reaction system generally increases the kinetic energy of the reactants, making it more likely that they will possess the activation energy to react. This explains why reactions typically proceed faster at higher temperatures.
- Catalysts:Catalysts are substances that lower the activation energy of a reaction without being consumed. By providing an alternative reaction pathway with a lower energy barrier, catalysts speed up reaction rates.
- Light:In photochemical reactions, light energy is absorbed by reactants, providing them with the activation energy necessary to react. This is commonly observed in reactions involving photosynthesis and the production of vitamin D in the human body.
Experimental Techniques
Determining the rate of a chemical reaction is crucial in understanding its kinetics and predicting its behavior. Various experimental techniques can be employed to measure reaction rates, each with its own advantages and limitations.
One common method involves monitoring the change in concentration of reactants or products over time. This can be achieved using spectrophotometry, titrations, or gas chromatography. Another approach is to measure the rate of gas evolution or heat release using techniques like manometry or calorimetry.
Designing an Experiment
To design an experiment to measure the reaction rate of a specific reaction, several steps should be considered:
- Identify the reaction of interest:Determine the specific chemical reaction to be studied and its reactants and products.
- Select an appropriate experimental technique:Choose a technique that is suitable for measuring the reaction rate based on the nature of the reaction and the available equipment.
- Control experimental conditions:Identify and control factors that may influence the reaction rate, such as temperature, concentration, and presence of catalysts.
- Establish a data collection plan:Determine the frequency and method of data collection to ensure accurate measurement of the reaction rate.
- Analyze the data:Use appropriate mathematical methods to analyze the collected data and determine the reaction rate.
Presenting Results
The results of the experiment can be presented in a table format to clearly display the data and facilitate analysis.
| Time (min) | Concentration of Reactant A (M) | Reaction Rate (M/min) |
|---|---|---|
| 0 | 1.00 | – |
| 10 | 0.80 | 0.02 |
| 20 | 0.64 | 0.016 |
| 30 | 0.51 | 0.013 |
Applications of Reaction Rates
Reaction rates are not just theoretical concepts but have immense practical applications across various fields, helping us understand and manipulate chemical processes in countless ways.
Industries such as pharmaceuticals, food processing, and energy rely heavily on reaction rates to optimize production, ensure product quality, and minimize environmental impact.
Pharmaceuticals
- Designing drugs with controlled release rates for targeted delivery.
- Monitoring drug metabolism and efficacy to optimize dosage and treatment plans.
- Understanding drug-drug interactions and minimizing adverse reactions.
Food Processing, Energy ws #1 reaction rates
- Controlling enzymatic reactions to preserve food quality and extend shelf life.
- Optimizing cooking times and temperatures to enhance flavor and nutritional value.
- Designing packaging materials that minimize food spoilage and contamination.
Energy
- Developing efficient fuel combustion systems for power plants and vehicles.
- Designing batteries with high energy density and fast charging rates.
- Understanding the kinetics of nuclear reactions for safe and efficient energy production.
Quick FAQs: Energy Ws #1 Reaction Rates
What are reaction rates?
Reaction rates measure the speed at which chemical reactions occur, indicating the change in concentration of reactants or products over time.
How does temperature affect reaction rates?
Increasing temperature generally increases reaction rates by providing more energy to overcome the activation energy barrier.
What is the role of catalysts in reaction rates?
Catalysts are substances that enhance reaction rates without being consumed, providing an alternative pathway with a lower activation energy.