Work equilibrium and free energy pogil – In the realm of thermodynamics, the concepts of work equilibrium and free energy reign supreme, dictating the spontaneity of reactions and guiding the behavior of complex systems. This article delves into the intricate relationship between these two pillars, exploring their significance and practical applications.
Work equilibrium, a state of balance where opposing forces cancel each other out, plays a pivotal role in determining the spontaneity of chemical reactions. Free energy, on the other hand, quantifies the potential for a system to perform work and serves as a reliable predictor of reaction feasibility.
Work Equilibrium and Free Energy: Work Equilibrium And Free Energy Pogil
Work equilibrium is a fundamental concept in thermodynamics that describes the state of a system in which the net work done on the system is zero. This occurs when the opposing forces acting on the system balance each other out, resulting in no overall change in the system’s energy.
Work equilibrium plays a significant role in determining the spontaneity and direction of chemical reactions.
Free energy is a thermodynamic potential that measures the maximum amount of work that can be extracted from a system at constant temperature and pressure. It is closely related to work equilibrium, as the change in free energy during a reaction is equal to the maximum work that can be done by the system.
The spontaneity of a reaction is determined by the sign of the change in free energy: a negative change in free energy indicates a spontaneous reaction, while a positive change indicates a non-spontaneous reaction.
Examples of Reactions at Work Equilibrium
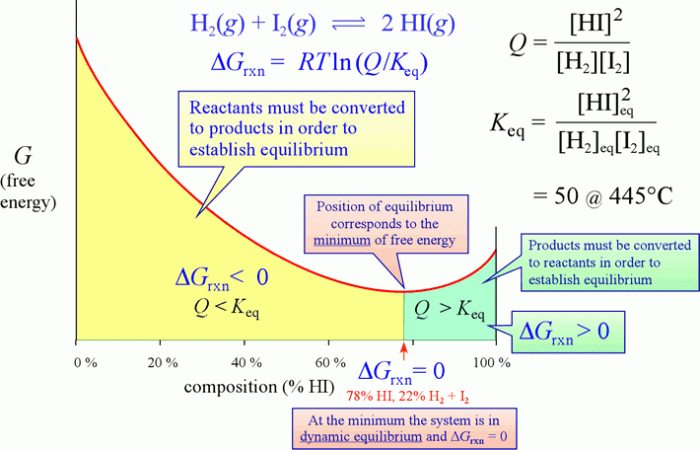
- The reaction of hydrogen and iodine to form hydrogen iodide is at work equilibrium at room temperature. The forward and reverse reactions occur simultaneously, with no net change in the concentrations of the reactants and products.
- The dissociation of water into hydrogen and hydroxide ions is also at work equilibrium. The equilibrium constant for this reaction is very small, indicating that the reaction strongly favors the formation of water.
Reactions at work equilibrium can be shifted by changing the conditions of the system, such as temperature, pressure, or concentration. For example, increasing the temperature of the hydrogen-iodine reaction will shift the equilibrium towards the formation of hydrogen iodide, while increasing the pressure will shift the equilibrium towards the formation of hydrogen and iodine.
Gibbs Free Energy
Gibbs free energy, denoted by G, is a thermodynamic potential that combines the enthalpy and entropy of a system. It is defined as the difference between the enthalpy (H) and the product of the temperature (T) and entropy (S):
G = H – TS
Gibbs free energy is closely related to work equilibrium. At constant temperature and pressure, the change in Gibbs free energy during a reaction is equal to the maximum work that can be done by the system:
ΔG = -W max
where ΔG is the change in Gibbs free energy and W maxis the maximum work.
Factors that affect Gibbs free energy include temperature, pressure, and concentration. Increasing the temperature will increase Gibbs free energy, while increasing the pressure or concentration will decrease Gibbs free energy.
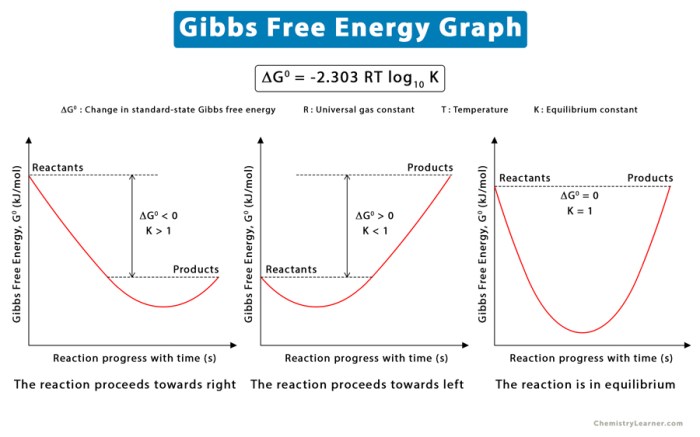
Graphical Representation of Gibbs Free Energy
Gibbs free energy can be represented graphically as a function of reaction progress. The graph shows the change in Gibbs free energy as the reaction proceeds from the initial state to the final state. The equilibrium point is the point at which the change in Gibbs free energy is zero.
The graphical representation of Gibbs free energy can be used to predict the spontaneity of reactions. If the change in Gibbs free energy is negative, the reaction is spontaneous and will proceed in the forward direction. If the change in Gibbs free energy is positive, the reaction is non-spontaneous and will not proceed in the forward direction.
Applications of Work Equilibrium and Free Energy, Work equilibrium and free energy pogil
Work equilibrium and free energy have numerous applications in various fields, including chemistry, biology, and engineering.
In chemistry, work equilibrium and free energy are used to:
- Predict the spontaneity of reactions
- Design and optimize chemical processes
- Understand the behavior of complex chemical systems
In biology, work equilibrium and free energy are used to:
- Understand the thermodynamics of biological processes
- Design and optimize biological systems
- Develop new drugs and therapies
In engineering, work equilibrium and free energy are used to:
- Design and optimize energy conversion systems
- Develop new materials
- Understand the behavior of complex engineering systems
Experimental Determination of Work Equilibrium
Work equilibrium can be experimentally determined using various methods, including calorimetry, spectroscopy, and other techniques.
Calorimetry is a technique that measures the heat released or absorbed during a reaction. The heat released or absorbed is related to the change in Gibbs free energy of the reaction.
Spectroscopy is a technique that measures the absorption or emission of electromagnetic radiation by a sample. The absorption or emission of electromagnetic radiation can be related to the change in Gibbs free energy of the reaction.
Experimental data on work equilibrium is essential for validating theoretical models of work equilibrium. By comparing experimental data with theoretical predictions, scientists can gain a better understanding of the factors that affect work equilibrium and how it can be used to predict the behavior of chemical reactions.
Clarifying Questions
What is the significance of work equilibrium in thermodynamics?
Work equilibrium establishes a state of balance where opposing forces cancel each other out, providing valuable insights into the spontaneity and feasibility of chemical reactions.
How does free energy determine the spontaneity of reactions?
Free energy serves as a quantitative measure of the potential for a system to perform work. A negative change in free energy indicates a spontaneous reaction, while a positive change suggests a nonspontaneous reaction.
What are some practical applications of work equilibrium and free energy?
These concepts find widespread applications in chemistry, biology, and engineering, guiding the design and optimization of processes, and enhancing our understanding of complex systems.